The Lab
At the Mikael Simons Lab we study the biology of glia and their interaction with neurons in health and disease. One focus is on oligodendrocytes and myelin, the primary target of the immune attacks in multiple sclerosis. We aim at identifying the mechanisms of how myelin is formed and regenerated following injury.
Another focus is on microglia, and their functions in homeostasis, aging and disease. We study the mechanisms of neuroinflammation and how microglia and other immune cells damage or restore nervous system function in neurodegenerative and inflammatory diseases.
The lab is part of the Institute of Neuronal Cell Biology, belongs to the TUM Faculty of Medicine, and is affiliated with the German Center for Neurodegenerative Diseases and the Institute for Stroke and Dementia Research. Our lab is located at the Center of Stroke and Dementia Research at Campus Grosshadern in Munich.
» Institute of Neuronal Cell Biology

Research
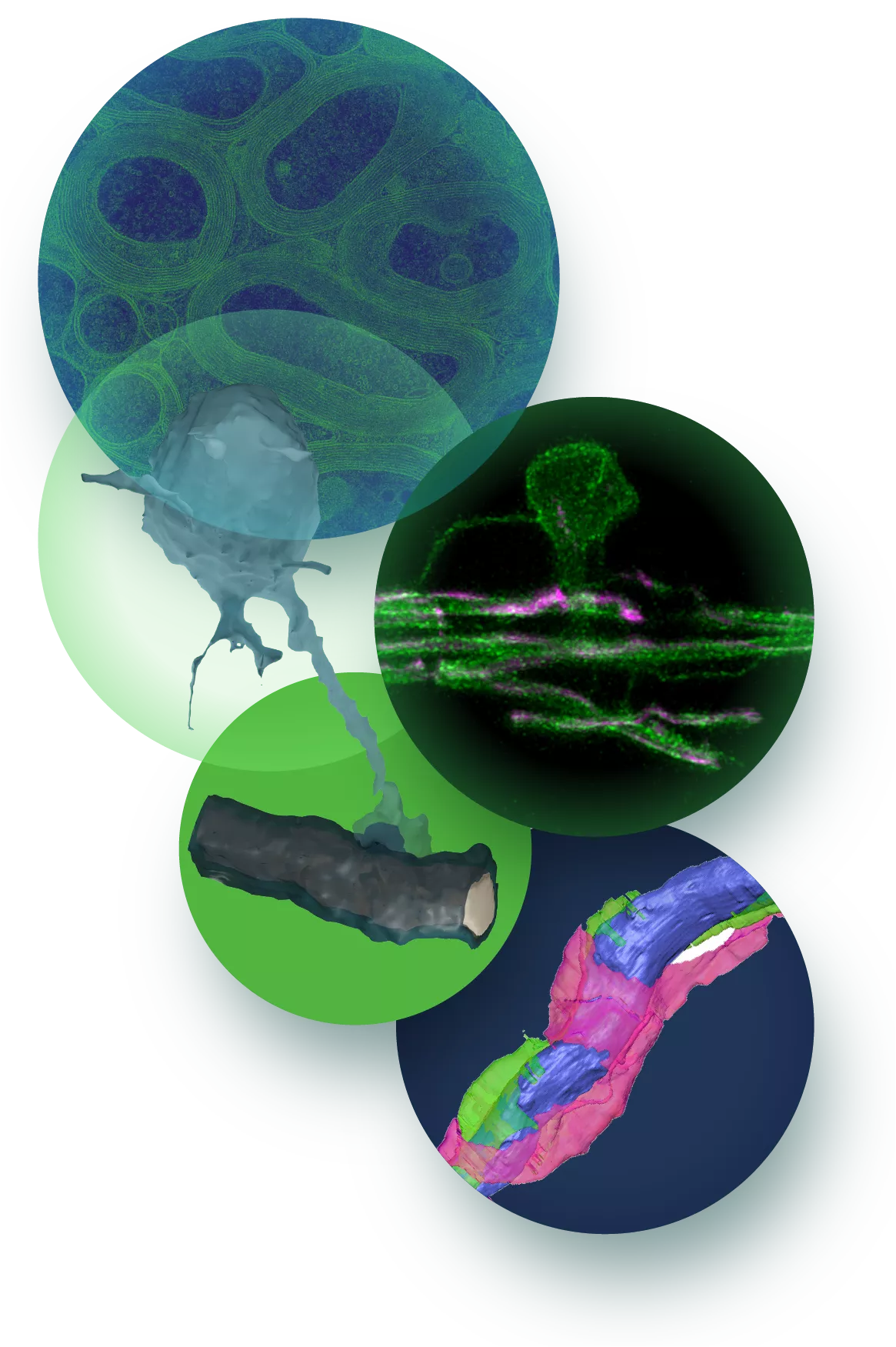
Development
Information processing in complex organisms requires fast nerve transmission in both the peripheral and central nervous system. This is accomplished by the ensheathment of axons with myelin to allow saltatory nerve impulse transmission. Our goal is to unravel the basic cellular and molecular processes of myelination and to understand how they are applied in the nervous system in vivo. We have previously developed a model that explains how myelin is generated as a multilamellar membrane stacks around an axon.
We now need to elucidate the contribution of neuron and oligodendrocyte interaction in myelin growth and wrapping. We study which neuronal factors determine whether an axon will be myelinated or not, how oligodendrocytes recognize and form stable contacts with the axons and the forces that drive the myelin sheath around the axon. Myelination does not only occur during development, but extends into adult life and contributes to brain plasticity being, modifiable by experience and various environmental factors.
We are studying adult-born oligodendrocytes and want to understand how they are actively engaged in forming new myelin sheaths. Since these processes have to be tightly coupled to the need of the neurons, we have to understand how oligodendrocytes and neurons communicate. As an experimental system, we use primarily mice, but also zebrafish and iPSC-derived human oligodendrocytes. Using a combination of genetics and imaging, we explore fundamental aspects of myelin cell biology in vivo. Our analyses include ultrastructural reconstruction of CNS tissue using a range of electron microscopy techniques (including high-pressure freeze TEM, FIB-SEM, ATUM-based array imaging). We complement our studies by mechanistic studies in cell culture and by in-vitro reconstitution.
Regeneration
Myelin disorders, such as multiple sclerosis, are among the most prevalent and disabling diseases in young adults. In multiple sclerosis, regeneration of demyelinated lesions can occur. However, on average only ~20% of the lesions display signs of regeneration.
We are studying how damaged tissue undergoes regrowth or renewal, leading to restoration of nervous system function. Our goal is to understand why myelin repair fails in multiple sclerosis and to develop regenerative medicines for the nervous system. A central obstacle for progress in this area has been the complex biology underlying the response to CNS injury. Acute CNS damage is followed by a multicellular response that encompasses different cell types and spans different scales. Once injury is triggered resident microglia and infiltrating macrophages are attracted toward the lesion.
After the inflammatory response has been built up, the pro-inflammatory reaction begins to subside, and the cells switch to a regenerative cellular phenotype and resolve from the lesions. The regenerative response is continued by oligodendrocyte progenitor cells, which start to proliferate and to migrate into the demyelinated lesion, where they differentiate into myelinating oligodendrocytes. Currently, we do not understand which factors determines lesion recovery.
Failure of inflammation to resolve is a key underlying reason of poor regeneration, and one focus is therefore on the biology of microglia during de- and remyelination, and their cross talk to other cells, in particular oligodendrocytes and the progenitor cells. In addition, we are exploring the link between lipid metabolism and inflammation, and its role in the regulation of regeneration. As an experimental system, we use primarily mice, but also zebrafish and iPSC-derived cell systems, and make use of single cell genomics and CRISPR/Cas9 based gene editing for functional screenings.
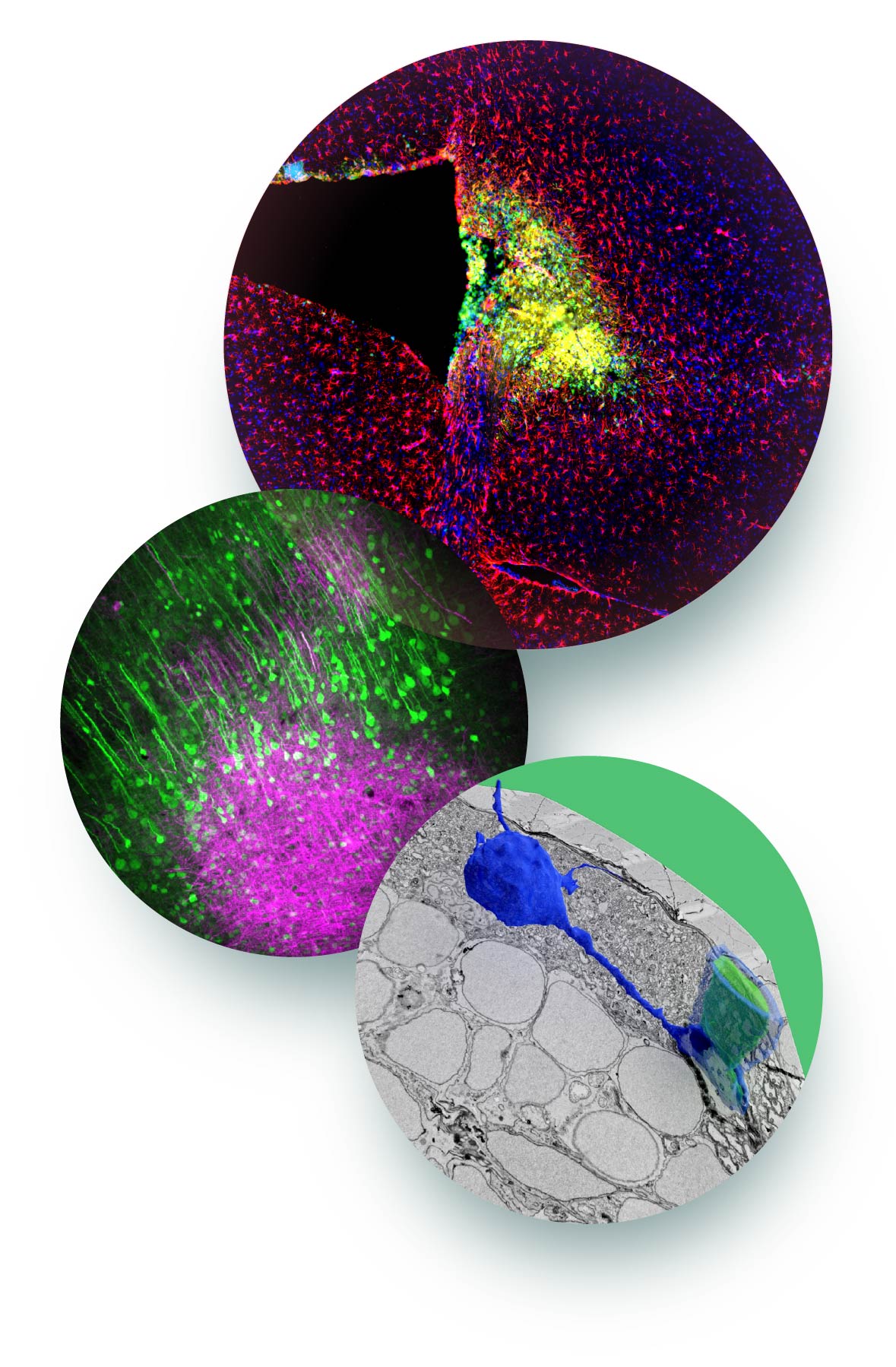
Aging/neurodegeneration
Age-related changes of brain structure are a major risk factor for a number of neurological diseases. White matter aging is associated with tissue shrinkage, and frequently also with focal lesions seen on magnetic resonance imaging as hyperintensities that are associated with cognitive impairment, and increased risk of stroke and dementia.
With age, a substantial number of myelin sheaths exhibit degenerative changes, and we have previously shown that these alterations lead to a specific response in microglia, that cluster into nodules, where they phagocytose myelin debris. This reaction may represents a protective response required to clear degenerated myelin accumulating during normal white matter aging.
However, the progressive accumulation of myelin debris may challenge microglia function with time so that the inflammatory response becomes chronic. We study the question of how myelin aging drives a chronic inflammatory response, and how inflammation is linked to the pathogenesis of age-related diseases.
We are also interested in the neuroprotective role of glia, in particular oligodendrocytes, in aging and neurodegeneration. To address these questions, we make use of single cell genomics, proteomics and lipidomics, and combine these analyses with functional studies in vivo by employing genetics and imaging techniques.

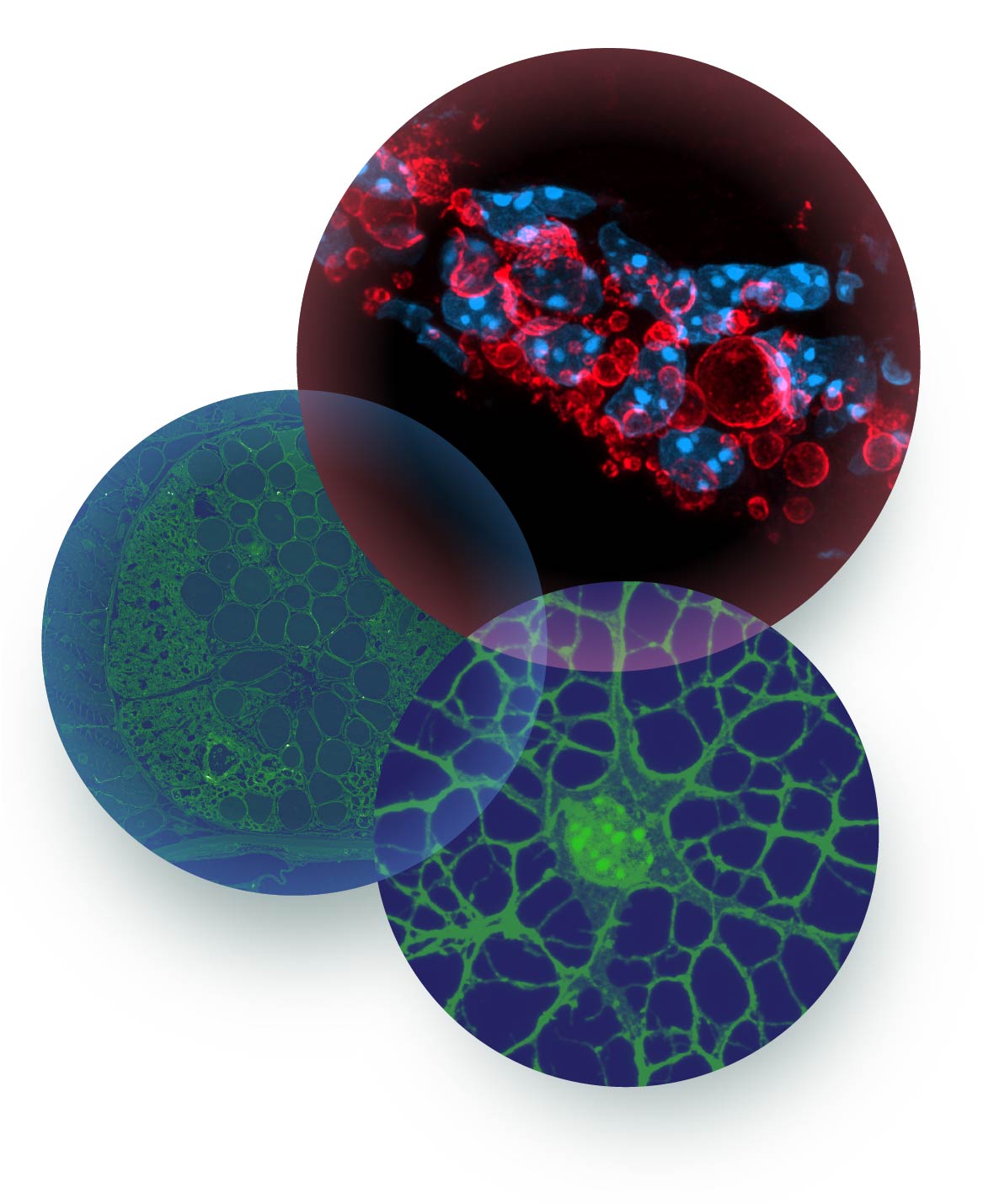
Lipoproteins
Due to the association of the apolipoprotein E4 variant with the development of late-onset sporadic Alzheimer’s disease, its functions is widely studied in the context of amyloid plaque formation. Yet, the fundamental cell and neurobiology of lipoproteins in the CNS is poorly understood.
We are interested in the functions of lipoproteins in development, regeneration, aging and neurodegeneration with the CNS. We are analyzing how lipoproteins function as vehicles in intercellular communication, and are exploring their functions as an extracellular surveillance and delivery system that connects lipid metabolic pathways between the different cells.
One important aim is to achieve a system-wide understanding of lipoprotein responses in aging and in neurodegenerative diseases, and to explore their protective, detoxifying functions, as well as their maladaptive, damaging roles. By employing an integrative and interdisciplinary approach aiming at combining genetics, biochemistry, cell biology and imaging, our goal is to obtain insights how lipid metabolism is linked to inflammation and neurodegeneration in the CNS.
Our Team
News
News Archive
Funding and Affiliation
- TUM – Institute of Neuronal Cell Biology » Visit
- DZNE - German Center for Neurodegenerative Diseases » Visit
- Clinical Trial Unit - DZNE Munich » Visit
- ISD - Institute for Stroke and Dementia Research » Visit
- SyNergy – Munich Cluster for Systems Neurology » Visit
- Single-cell Transcriptome Hub » Visit
- Electron Microscopic Hub » Visit
- CRC 128 – Collaborative Research Center 128 » Visit
- CRC 274 – Collaborative Research Center 274 » Visit
- Adelson Medical Research Foundation » Visit
- Chan Zuckerberg Initiative » Visit
- MSc. Biomedical Neuroscience » Visit
- GSN – Graduate School for Systemtic Neurosciences » Visit


























